

Two analytical chemists from the University of Notre Dame won poster awards at the Analytical Chemistry section of the International Chemical Congress of Pacific Basin Societies in Honolulu, Hawaii. Nicholas Myers, a graduate student in the laboratory of Marya Lieberman, associate professor of chemistry and biochemistry, and Xin Gu, a graduate student in the lab of Jon Camden, associate professor of chemistry and biochemistry, received awards.
The competition is very competitive. Out of 3,645 poster presentations, 350 were nominated for the poster competition, and the top 54 posters received awards. Myers presented a poster titled, "A paper test card for detection of substandard beta lactam antibiotics" in the Analytical Chemistry section of Pacifichem. The poster authors include Nicholas Myers, Jamie Luther, Doa’a Aldulaimi, and Marya Lieberman. Also in the Analytical Chemistry section, Gu presented a poster titled, “Plasmonic nanoprobes for sensing hydrogen peroxide in living systems.” Hubert Turley and Sarah Griffin also in the Camden lab presented posters as well.
Further explanation of Myers work:
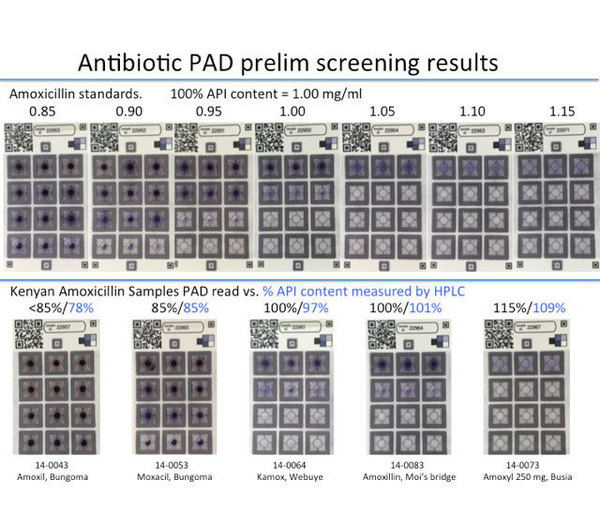
Figure 1: Top row: standard samples of amoxicillin. Bottom row: amoxicillin capsules collected in Kenya in 2014. The antibiotic prevents dots on the card from turning blue. If the pill does not have enough antibiotic, more than six dots will turn blue. The test results were checked using high performance liquid chromatography (HPLC).
Finding low-quality pharmaceuticals in the developing world can be challenging because assaying finished pharmaceutical pills usually requires a sophisticated analysis in a lab setting. We performed HPLC analysis on pills containing amoxicillin, clavulanic acid, and ampicillin which were collected in Kenya. About 20% (n = 90) did not meet regulatory specifications, and the pills that failed analysis spanned the entire range of 0-150% of the labeled active ingredient. Having a portable and inexpensive paper-based test card that can analyze beta-lactam antibiotics over this range could help monitoring agencies perform market surveys or even empower manufacturers to do more quality control testing.
The United States Pharmacopeia <425> describes the use of an iodometric back-titration to quantify amoxicillin and ampicillin. The back-titration was translated onto a paper test card. The titration reagents, which are mutually incompatible, could be stored separately and recombined through surface-tension enabled mixing. Good distinction is achieved on the test card by amoxicillin solutions that differ by 0.15 mg/mL over the range of 0-0.9 mg/mL. This means a pill containing 80% amoxicillin can be differentiated from a pill containing 100% amoxicillin. This new test card will be trials in Kenya in early January to see how well it works in the field.