The second annual Rare Patient Advocacy Summit was held on September 16, 2022 in the Jordan Hall of Science. The College of Science welcomed the various stakeholders in raising awareness to the challenges and practices in rare disease advocacy. The summit highlighted voices from biotechnology, public policy, and most importantly, patient families whose experiences shed a new light on the real need for patient advocates in the field. The Rare Patient Advocacy Summit was sponsored in part by Dyne Therapeutics and Horizon Therapeutics, the College of Science and the Minor in Science and Patient Advocacy. CEO of Dyne Therapeutics, Josh Brumm, opened the summit by declaring his commitment to advocacy and ensuring all Dyne employees are active patient advocates that deliver results by working with patient families through the process of clinical trial developments and treatment approval.
The summit included three panels composed of various stakeholders and experts. First and foremost, the Patient Perspectives panel, featured patients and their families as experts on Huntington’s disease, Myotonic dystrophy (DM1), Duchenne Muscular Dystrophy (DMD), and Facioscapulohumeral Muscular Dystrophy (FSHD). The panel, moderated by Molly White, explored the challenges that patient families have when advocating for their specific challenges and navigating clinical trials and drug development.
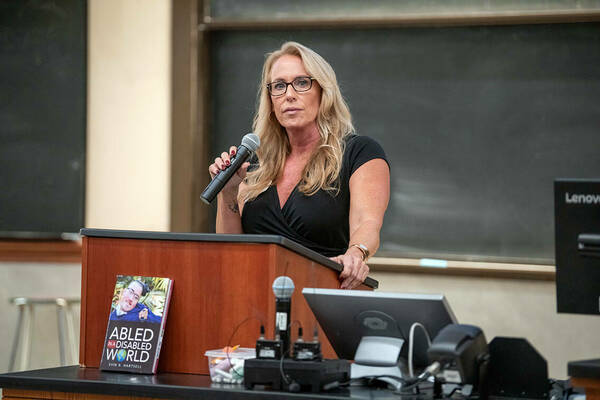
Two guest speakers, Melisa and Scott Hartsell shared the family history of their son, Evan Herstell, who lived with DMD. Prior to his death, Evin fought for patient advocacy by writing a book called Abled in a Disabled World. From his own experiences, he discusses the challenges people with disabilities face and the need to improve accessibility in our communities. Most of the burden of advocacy falls to patients and their families, and those involved in this work aim to change that.
Barbara Calhoun, Director of the Minor in Science and Patient Advocacy, works with various community partners to provide students with the necessary skills to effectively advocate for patients. The ultimate goal of the minor is to impress the practices of effective advocacy in the discipline of future healthcare professionals from science-business to healthcare. Calhoun says, “By listening to patient families, who are the experts in their disease, we can integrate their needs and knowledge to enhance the success of all aspects of rare disease research and drug development from clinical trials to approval.”
In the panel Patient Advocacy in Biotech and Pharma, guests heard directly from the professionals making impact in biotechnology and pharmaceutical programs. Cindy Parseghian, from the Ara Parseghian Medical Research Fund, advocated for the continued funding of research as an essential element in expediting the treatments policies for rare diseases. BJ Viau, Horizon Therapeutics; Molly White, Dyne Therapeutics; John Crowley, Amicus Therapeutics; Cindy Parseghian, Ara Parseghian Medical Research Foundation; moderated by Tom Hamilton, Larimar Therapeutics, Friedrich’s Ataxia Research Alliance also engaged in this conversation.

The final panel, Trends and Challenges: Clinical Trials and Drug Approval was moderated by Sean Kassen, Ara Parseghian Medical Research Fund. Dr. Kassen interviewed Anju Samy, Horizon Therapeutics; Tim Franson, Faegre Drinker Consulting; Dr. Elizabeth Berry Kravis, Rush University Medical Center; and Debra Feldman, Dyne Therapeutics. Physicians conducting clinical trials and the experts that work with regulatory agencies such as the FDA, discussed how they successfully navigate the process of approving treatments. Inclusion of patient and family perspectives inform and influence the efficiency of clinical trials and treatments.
Tim Walbert, CEO of Horizon Therapeutics closed out the summit with a moving testimonial on his own experience in living with a rare disease. Notre Dame is grateful for the generous support from Horizon Therapeutics as well as the support from Dyne Therapeutics.These new partnerships support the efforts of making patient advocacy part of the conscious practice for all stakeholders working in the rare disease and patient advocacy.
Originally published by at spaminor.nd.edu on September 25, 2022.