In the alcove area outside Stepan and Nieuwland halls, University of Notre Dame students gather weekly to boil up batches of brew.
The wind chill clocked in at 18 degrees during the first week of classes, but science and engineering students in Chemistry 40426 quickly lit propane burners and filled stainless steel kettles to ferment a formula that will transform into beer within four weeks.
The lab is part of Professor Masaru Kuno’s course, The Chemistry of Fermentation and Distillation. Kuno published a book on the course, called Introductory Science of Alcoholic Beverages, and it’s also not your average “how to make booze” book, because it’s all based on chemistry.

As he worked with his group around a boiling kettle, helping pour a sticky, thick malt extract into the water, Daniel Diejomaoh, a senior science business major, said he enjoys learning about a wide range of topics but knew this course would be special.
“Having a class like this, where we have already learned about organic chemistry and chemistry, is good because instead of just learning the information, we actually get to use it,” he said. His friends who aren’t in the class (of legal drinking age) will get to partake in a taste test of all the products, and he’ll share the results as part of a video presentation at the end of the semester.
To take the course, students should have taken at least one year of chemistry courses. However, he doesn’t assume knowledge of organic chemistry other than to show chemical structures and describe important impurities in alcoholic beverages that render their unique characteristics and flavor profiles.
“I want folks to be able to not only learn the theoretical side of things, but I also want them to experience what fermentation looks like in real life,” Kuno said. “I want to show them the ins and outs of what beverage production looks like.”
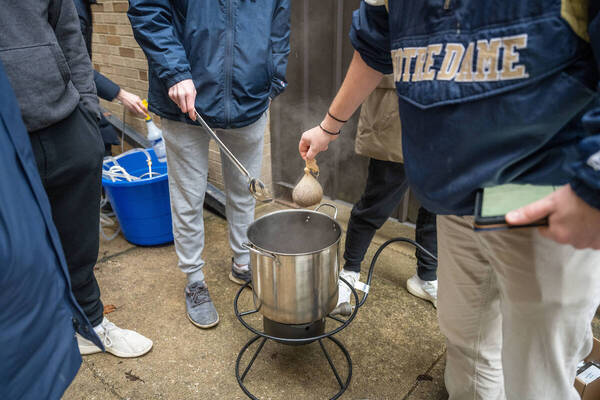
The classroom is “flipped,” meaning Kuno provides the lectures in video format and the students attend class just for the experiments. They started brewing beer during the first week of class, but will also make wine.
Kuno inherited the class in 2017 from Dan Gezelter, professor in the Department of Chemistry and Biochemistry, who is now associate dean for undergraduate studies. Holly Goodson, a professor in the department, and James Parise, former teaching professor at Notre Dame, also taught the class in the past.
Kuno said he’s more of a diet Coke drinker than a wine or beer connoisseur, acknowledging that he learned a lot about the field of brewing alcoholic beverages while preparing to teach the class. He was surprised to discover that the difference between type and taste of different alcoholic beverages mainly came from chemical impurities.
“Yeast produces metabolic byproducts, which are common to everything, so you have a base, aroma, and flavor profile for many beverages,” Kuno said. “So there’s a common lineage that connects wine, spirits, and even beer.
“If we just ignored the impurities, everything would taste just like watered-down ethanol, which is like a very watery vodka,” he said.
For those who can’t take the class, the concepts Kuno teaches are included in the book, which details all the chemical processes for fermentation, beer, wine, spirits, and distillation.

The labs for the class, which is capped at 35 students (and always full), are completed outside because there’s not a dedicated, food-safe laboratory inside any of the buildings. But students don’t mind, even as they huddle as close to the burners as possible while waiting for the chemical “magic” to happen.
“I took a food science class (at Notre Dame) last year, which was super fun, and we only learned a little about fermentation,” said Christina Vail, a senior chemical engineering major. “But I really wanted to take this class; it’s fun to do with a bunch of your friends, especially senior year, and puts the joy back in school.”